AAAR 2023 Poster
Nitrophenols are Photoacids?
TLDR
The ultrafast dynamics of 4-nitrocatechol suggest highly-efficient proton transfer in the excited state. This process occurs within a few picoseconds in water. Interestingly, this process also occurs in 2-propanol, a less efficient proton acceptor. These excited-state proton transfer processes may be the reason why 4-nitrocatechol has low photochemical yields.
Motivations
Nitrophenols are strong light-absorbers in both the UV and Visible. This gives NPs the ability to absorb sunlight and undergo chemical transformations in the atmosphere.
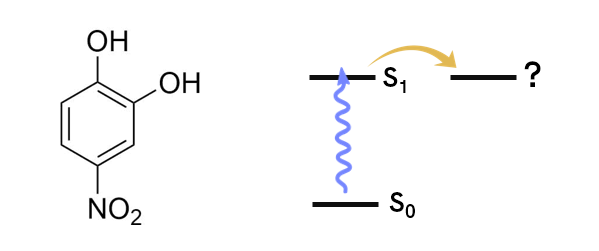 Fig. 1. Molecule used (4NC) and simplistic Jablonski diagram
Fig. 1. Molecule used (4NC) and simplistic Jablonski diagram
They are known to photodegrade, but the electronic states responsible for photochemical reactions are not well characterized.
Methods
Pump-probe experiments were conducted with a 340 nm pump pulse. Transient absorption was measured by varying the time delay between pump and probe pulses with a delay line. Spectra were produced via a spectrograph with a CCD detector.
 Fig. 2. Experimental setup
Fig. 2. Experimental setup
Theoretical calculations
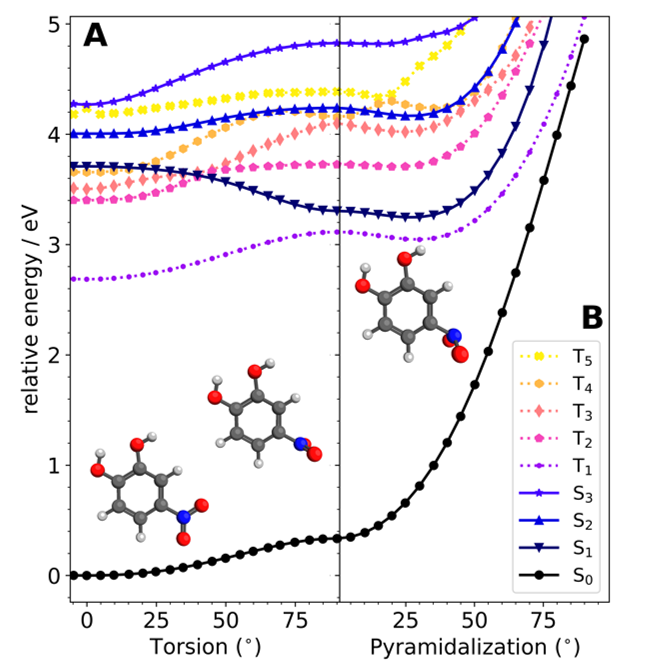 Fig. 3.Energy surfaces calculated with TD/TDA-PBE0/6-311+G(d)
Fig. 3.Energy surfaces calculated with TD/TDA-PBE0/6-311+G(d)
Density functional theory calculations suggest that 4NC may reorient itself in the first excited singlet state (S1).
Solvent-dependent dynamics
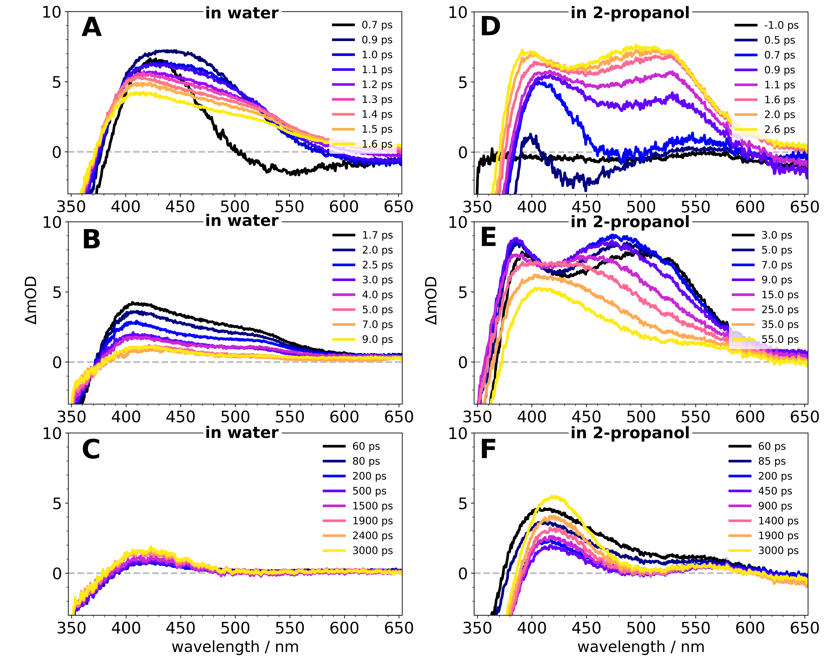 Fig. 4. Transient absorption spectra in water and 2-propanol
Fig. 4. Transient absorption spectra in water and 2-propanol
Timeline in Water
1.) Sub-ps molecular rearrangement (twisting)
2.) Deprotonation around 1-2 ps
3.) Relaxation of the anion
4.) Long-term build-up of anion in solution
Timeline in 2-propanol
1.) Sub-ps twisting
2.) Intersystem crossing (~0.7-2.6 ps)
3.) Triplet population maxes out around 7 ps
4.) Deprotonation happens from triplet state (~9-35 ps)
5.) Relaxation of the anion
6.) Long-term build-up of anion in solution
Excited-state proton transfer in both solvents!
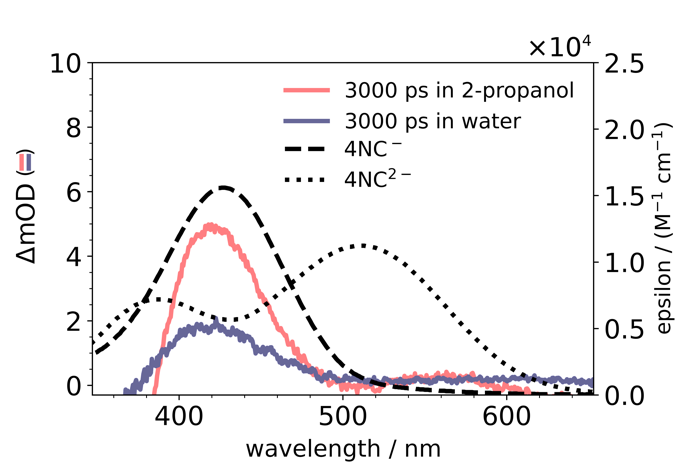 Fig. 5. Transient absorption and molar extinction spectra
Fig. 5. Transient absorption and molar extinction spectra
The transient absorption spectra at long delay times resemble the absorption by the ground-state anion 4NC−. The spectrum is less pronounced in water due to the fact the water is more likely to re-protonate 4NC− than 2-propanol.
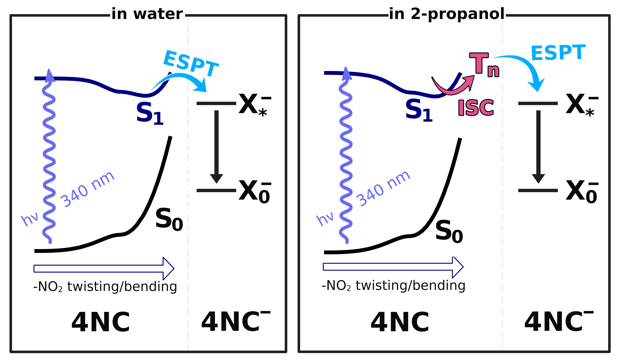 Fig. 6. Jablonksi-like diagram summarizing observed dynamics
Fig. 6. Jablonksi-like diagram summarizing observed dynamics
Although both solvents appear to allow for 4NC to undergo excited-state proton transfer, the pathways seem to differ in the two solvents.